UNLOCK YOUR INNER SELF
The future of mental health and personal development is in psychedelic medicine. We are on a mission to create innovative drug formulations for the treatment of anxiety, depression, and pain management.
Our Vision
industry leading
We are a Canadian bio-pharmaceutical and biotechnology company comprised of a best of class sciences and executive leadership team with access to patents and capitals markets experience. We will use our psilocybin and psilocin research and development plan to develop evolving life sciences of psilocybin and psilocin drugs for clinical trials and patient prescribed uses globally.
Our Vision
industry leading
We are a Canadian bio-pharmaceutical and biotechnology company comprised of a best in class sciences and executive leadership team with access to patents and capitals markets experience. Our psilocybin and psilocin research and development program is designed to create innovative psilocybin and psilocin drugs and protocols for clinical trials and patient prescribed uses globally.
The Future of Psychedelic Medicine
Cube Accelerated Treatment Technology (CATT)
The goal of Cube Psytech is to support and provide cost-effective and reliable solutions for people suffering from mental disorders and addictions.
Artificial Intelligence (AI) Assisted Psychotherapy
Cube Tech is a specially designed global date driven AI platform that enables us to assess and identify the deviations from optimized healthy lifestyle data, to create new protocols.
Psilocybin Assisted Psychotherapy
Cube Psytech team has identified psilocybin-assisted psychotherapy and clinical studies that can demonstrate efficacy of potential disease- modifying therapeutics to help combat Cancer-Related Psychiatric Distress, Addiction, Treatment-Resistant Depression, Obsessive- Compulsive Disorder, Cluster Headaches.
A Message From Our Founder

"Our vision is to create an industry leading bio-pharmaceutical and biotechnology company focused on psilocybin and alternative treatment options for patients around the world. We have assembled a top notch science team with over 10 combined PhDs, and a proven leadership team with over 50 combined years of senior management experience. A true collaboration of gifted minds in both science and business. ”
F O U N D E R A N D C E O
Investor Relations
best of class sciences and executive leadership team
The company is comprised of a best-in-class science and executive leadership team with access to patents and capitals markets experience. The team has proven track records of starting up and growing new Health Canada regulated business’s that are focused on controlled substances.
- World class drug delivery and formulation R&D Science Team
- Accessibility for 10+ patents on precision drug delivery systems
- Access for 1902 total publications on psychedelics of 1239 psilocybin, up to date
- Expedited CSE and OTC Markets Dual Listing Go Public Plan
- Proven Capital Markets Experiences
- Land and Building Assets
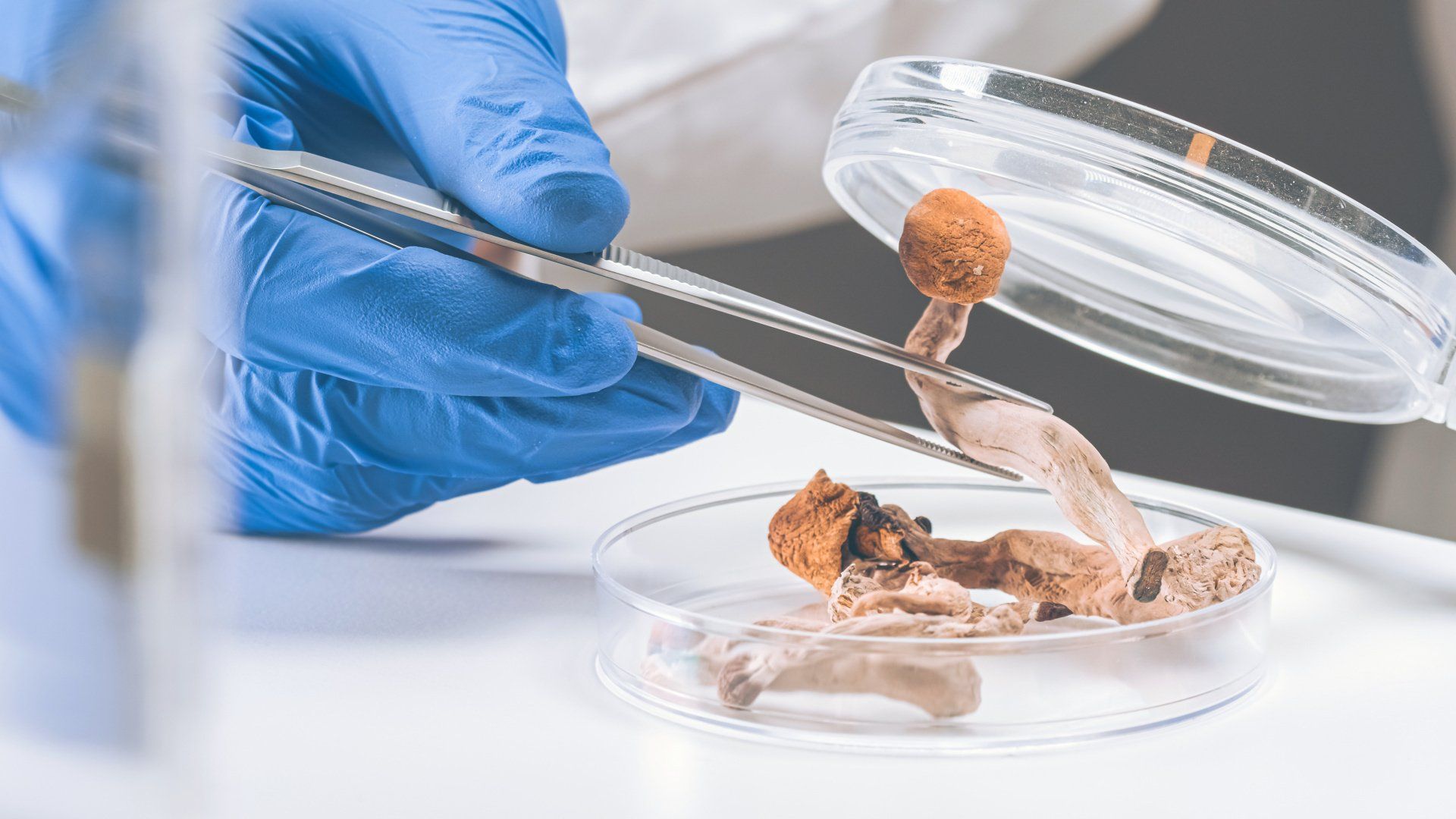
- Functional Mushroom Line
Investor Relations
best of class sciences and executive leadership team
Proven track records of starting up and growing new Health Canada regulated business’s that are focused on controlled substances.
- World class drug delivery and formulation R&D Science Team
- Accessibility for 10+ patents on precision drug delivery systems.
- Access for 1902 total publications on psychedelics of 1239 psilocybin to date.
- Expedited CSE and OTC Markets Dual Listing Go Public Plan.
- Proven Capital Markets Experiences.
- Land and Building Assets.
- Functional Mushroom Supplement Line launching 1st quarter 2022.
Press Releases


News

Psychedelics may be the "mental health fix we've been looking for," according to a state legislator.
Our Products
NATURAL FUNCTIONAL MUSHROOMS PRODUCTS
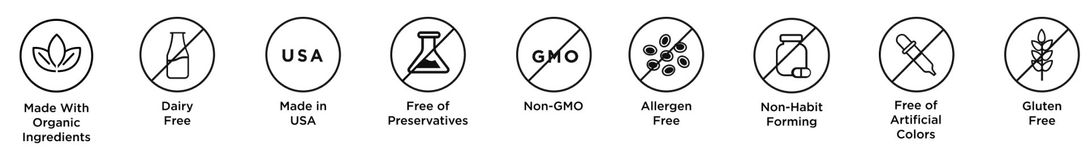
Our non-psychoactive line up of products features formulations that include healthy functional mushrooms and supportive compounds chosen to target our modern health issues. We use ingredients that balance the best nature with the confidence of science. All our products are Non-GMO - FDA Compliant - Drug Free - Gluten & Allergen Free - Non-Habit Forming - Free of Artificial Colours, Flavours and Preservatives.

On A Mission
We created products to help you with alleviating the pressures of modern life. Our formulations are based on the science of proven ingredients.

Formulated With Care
We introduce products when we feel they meet our strict principles of quality, transparency, and efficacy. We will never claim to have the widest assortment; others can stock thousands of products. Our curated selection allows us to be deliberate with regards to scientific backing and ingredient supply chain. It allows us to ensure all of our products are properly tested and verified.
All Rights Reserved | Cube Psytech